Abstract
Background: Splenic infiltration and associated splenomegaly is a challenging complication of non-Hodgkin lymphoma (NHL). The choice between splenectomy and splenic radiation (RT) is controversial, with little data to provide guidance in NHL. We aim to assess the tolerability and efficacy of splenic RT in NHL.
Methods: We reviewed NHL patients treated from 2002 to 2021 with RT to the spleen alone. Splenomegaly was defined as vertical length >13cm. Splenic volume was obtained by manual contouring in MIM PACS 6.9.7 on the baseline pre-RT CT scan and the CT scan at best splenic response post-RT. PET response was evaluated by Lugano criteria. Adverse events (AEs) were retrospectively graded by clinicians using CTCAE v5.0. Cell counts were collected at baseline pre-RT and through 90 days post-RT completion; cell counts were censored at initiation of cytotoxic chemo post-RT if received. Significance was assessed with the Kruskal Wallis test.
Results: 21 patients (11 diffuse large B-cell lymphoma (DLBCL), 5 marginal zone lymphoma (MZL), 4 mantle cell lymphoma (MCL), 1 follicular lymphoma) were identified with 23 courses of splenic RT. Median age was 75 (42-94); median KPS was 80 (50-90). Most patients (57%) had splenic only disease. Median splenic height pre-RT was 13.6 cm (6.4-26.6); 13 patients (57%) had baseline splenomegaly. Median splenic volume was 682cc (104-3355). 11 patients (48%) were symptomatic pre-RT (most frequently with pain: n=7, dyspnea: n=4, or nausea: n=4). 91% had any cytopenia before RT with median baseline absolute neutrophil count (ANC) 2.3 (0.2-10.6), hemoglobin 9.8 (7.1-13.8), and platelets 93 (9-221). RT was performed with definitive (n=11) or palliative (n=9) intent, or as bridging to chimeric antigen receptor T cells (n=3). Median dose was 30Gy for DLBCL (10.8-45), and 4Gy for MCL/MZL/FL (0.6-20); 10 patients received ≥20Gy.
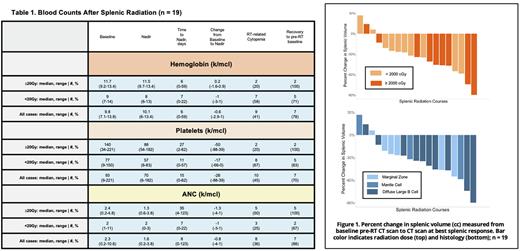
RT was well tolerated with 43% of courses without AEs and 57% with G1-2 AEs. AEs with frequency >10% were G1 fatigue (n=6), G1 anorexia (n=5), G1 nausea (n=5), G1 diarrhea (n=4), and G2 fatigue (n=4). There were no G3-4 AEs. One patient died from respiratory failure secondary to fungal pneumonia which was not felt to be attributable to splenic RT. Median nadirs in the 19 patients with cell count data: ANC 1.6 (0.2-3.8), hemoglobin 10.1 (6-13.4), platelets 70 (6-182) with 8, 6, and 15 median days from RT start to nadir (Table 1). ANC and platelets were disproportionately affected (median reduction: 26% and 28% for ANC and platelets, respectively vs. 5% for hemoglobin; p=0.006). There were no significant differences in reductions in ANC, platelets, or hemoglobin by RT dose (≥20Gy vs. <20Gy; p>0.1). In 3 patients (14%) cytopenias completely normalized post-RT. In most patients, cell counts normalized to pre-RT baseline (see Table 1). 4/13 symptomatic patients (31%) experienced complete relief. Median reduction in splenic volume was 34% (0-90%) with no significant difference in cytoreduction between low and high dose RT groups (median 27% reduction in <20Gy and 46% in ≥20Gy; p=0.2), see Figure 1. Overall response rate (ORR) by PET was 85% (35% complete response, 50% partial response, 10% stable disease, 5% progressive disease). With a median follow up of 12mo, median duration of radiographic response was 8mo (0.5-103) with more durable response at ≥20Gy (median 35 vs. 4mo, p=0.01).
Conclusion: Splenic RT was well tolerated and had a high ORR of 85%. Radiographic response was more durable at doses ≥20Gy. Larger sample sizes with homogenous histology are needed to further evaluate the impact of dose on cell counts, clinical toxicity, and symptomatic relief.
Disclosures
Imber:GT Medical Technologies: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal